Author Archives: robyn
Ampelopsis aconitifolia
 Download PDF
Download PDF
Name: Ampelopsis aconitifolia Bunge.
FAMILY: Vitaceae (the grape family)
Common Names: Monk’s-hood-vine, monkshood vine (1,2).
Etymology: The genus Ampelopsis is derived from from the Greek word ampelos, for ‘vine’. The epithet aconitifolia is likely a combination of Aconitum and folium (11), referring to the similarity of the leaves of A. aconitifolia to the leaves species of the genus Aconitum (Ranunculaceae).
Botanical synonyms: Vitis aconitifolia (9).
Quick Notable Features:
¬ Liana with 2-3 branched tendrils opposite the leaves
¬ Palmately compound leaves with pinnately lobed leaflets
¬ Cymose inflorescence of greenish flowers
¬ Yellow to orange round berry
Plant Height: Up to 12 m (9).
Subspecies/varieties recognized (1):
A. aconitifolia var. aconitifolia
A. aconitifolia var. cuneata Diels & Gilg
A. aconitifolia var. dissecta (Carrière) Koehne
A. aconitifolia var. glabra Diels & Gilg
A. aconitifolia var. palmiloba (Carrière) Rehder
A. aconitifolia var. setulosa Diels & Gilg
A. aconitifolia var. tomentella Diels & Gilg
Most Likely Confused with: Ampelopsis brevipedunculata, Vitis ssp., Parthenocissus ssp., Aconitum napellus, Cannabis sativa.
Habitat Preference: Although largely a cultivated species in Michigan, it can grow in thickets and forest edges. Monkshood vine prefers open habitats, and in its native range it is found in grasslands, valleys, and shrublands (2,5).
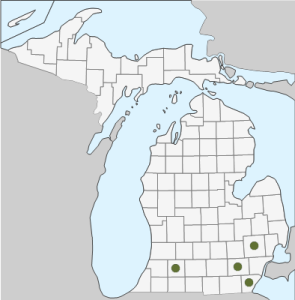
Geographic Distribution in Michigan: The Monk’s-hood-vine has been collected outside of cultivation only in Wayne County (2).
Known Elevational Distribution: In China, A. aconitifolia grows to elevations of 2200 m (5).
Complete Geographic Distribution: Native to northern China and Mongolia. In the United States, A. aconitifolia has escaped from cultivation in MI, NC, NJ, OH, PA, and New England. The species is also found in eastern Europe, in the Carpathian Mountains (2,3,6,12).
Vegetative Plant Description: A. aconitifolia is a fast growing, deciduous woody vine with tight bark bearing longitudinal ridges. The tendrils are 2-3 branched and borne opposite the leaves. The thin stipules are glabrous or slightly pubescent, brownish, approximately 2.3 mm long, 1-2 mm broad, and apically obtuse. The petioles are also glabrous, or slightly pubescent, and 1.5-2.5 cm long. The alternate leaves are palmately compound with 3 or 5 nearly sessile, pinnately lobed leaflets with irregularly toothed margins. The leaflets are lanceolate to rhomboid, apically acuminate, basally cuneate, 4-9 cm long, 1.5-6 cm broad, and sparsely pubescent below (2,5,9).
Climbing Mechanism: Monkshood vine uses its branched tendrils to climb (9).
Flower Description: The cymose inflorescence of A. aconitifolia is borne on a glabrous or slightly pubescent peduncle (1.5-4 cm long), opposite to the leaves, or less often borne on the tendrils. The nearly glabrous pedicels bear inconspicuous, greenish, perfect flowers developing from ovoid buds. The calyx is 5-parted and glabrous. The corolla is glabrous and composed of 5 oval petals 1.7-2.7 mm long. The 5 stamens have oval anthers. The ovary is superior, 2-locular, with 2 ovules per locule, and adnate to a well-developed floral disk. The style is cone-shaped, with a simple stigma (2,5,9).
Flowering Time: In Missouri, it flowers from July to August (5).
Pollinator: In the genus Ampelopsis, the floral disk is well developed and produces nectar (5,8), which may attract insects for pollination. However, no literature on specific pollinators has been found.
Fruit Type and Description: The round berries (6-8 mm in diameter) are yellow to orange and produce 1-4 (most commonly 2-3) seeds (2,5,9,14).
Seed Description: The globose seeds of A. aconitifolia are brown, 4-9 mm long, and 3.7-6 mm broad. The seed coat (testa) is reported as smooth or granular (6,13). The “chalazal knot [is] rounded, raphe [is] attenuate, ventral holes [are] furrowed upward 1/3 from base” (5).
Dispersal Syndrome: The fruits of A. aconitifolia are consumed by birds capable of seed dispersal (14).
Distinguished by: Ampelopsis brevipedunculata has simple leaves, often palmately lobed, and its berries are speckled and bright blue. The leaves of A. aconitifolia are palmately compound, leaflets pinnately lobed, and its berries are yellow to orange. Vitis ssp. (grapes) have a characteristic shredding and peeling bark, the leaves are simple, the corolla is apically fused, and the berries are dark bluish to black. A. aconitifolia has tight bark, and the corolla is free. Parthenocissus spp. have unlobed and regularly toothed leaflets, the tendrils are 4-12 branched and may end in adhesive disks (especially in P. quinquefolia), and the fruit is dark blue-black. In A. aconitifolia, the leaflets are pinnately lobed and irregularly toothed, and the tendrils are 2-3 branched and never end in adhesive disks. Aconitum napellus (garden’s monkshood) is an erect herb with no tendrils. The leaves are similarly shaped (belonging to the genus for which the species A. aconitfolia is named), but the flowers are blue, zygomorphic, conspicuous, and shaped like a “hood”, later developing into 3 follicles. A. aconitifolia is woody and climbing, with greenish, actinomorphic, inconspicuous flowers, later producing a berry. Cannabis sativa (marijuana) is an erect herb with palmately compound leaves and up to 11 leaflets (3 or 5 in A. aconitifolia) with unlobed serrate margins. The flowers of C. sativa are unisexual (perfect in A. aconitifolia) and female flowers produce an achene (2,5).
Other members of the family in Michigan (number species): Ampelopsis (1), Parthenocissus (2), Vitis (4) (source 2).
Ethnobotanical Uses: Monk’s-hood-vine is used as an ornamental plant (6), however medical uses or plant edibility were not found in the literature.
Phylogenetic Information: The genus Ampelopsis is included in the Vitaceae family, in the Vitales order of Core Eudicots. Vitaceae is the only family in the order Vitales, and it is subdivided into two subfamilies: Leeoideae and Vitoideae. All members of the family native to North America (including grapes – Vitis ssp.) and Ampelopsis are from the Vitoideae subfamily (4). The genus Ampelopsis has “about 30 species: Asia, Central and North America, with most species in E. Asia and two species in SW Asia; 17 species (13 endemic) in China” (5). The genus is paraphyletic and divided into two subclades: pinnately leaved (sect. Leeaceifoliae), and simple or palmately leaved (sect. Ampelopsis). A. aconitifolia is within the simple or palmately- leaved subclade along with 5 other species, including A. brevipenduculata, its closest relative. Section Ampelopsis is closer to the genus Rhoicissus. Soejima & Wen (2006) conclude that “both morphological and phylogenetic data suggest that Ampelopsis needs to be redefined and the “Leeaceifoliae” group may need to be raised to the generic rank” (8).
Interesting Quotation or Other Interesting Factoid not inserted above: A. aconitifolia var. glabra is reported as invasive by the Missouri Botanical Garden (7), and should be avoided as a gardening choice. A study in New England estimates that the species is potentially invasive there due to its similarity to the invasive Ampelopsis brevipenduculata (12,14). Erysiphe necator, a fungus that causes powdery mildew in many members of Vitaceae and one of the most damaging pests in California’s vineyards, also infects and damages A. aconitifolia (10).
Literature and websites used:
- Tropicos.org. Missouri Botanical Garden. 02 Jan 2014 <http://www.tropicos.org/Name/34001557>
- Michigan Flora Online. A.A. Reznicek, E.G. Voss, & B.S. Walters February 2011. University of Michigan. Web. January 2, 2014. http://michiganflora.net/species.aspx?id=2801.
- USDA, NRCS. 2013. The PLANTS Database (http://plants.usda.gov, 12/02/2013). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- Stevens, P.F. Angiosperm Phylogeny Website. Version 12, July 2012. http://www.mobot.org/mobot/research/apweb.
- Chen, Z. & J. Wen 2007. Flora of China, Vol. 12. 7. Vitaceae: 3. Ampelopsis. Web. http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200013477
- Bojňanský, V. & A. Fargašová 2007. Atlas of Seeds and Fruits of Central and East-European Flora: The Carpathian Mountains Region. The Netherlands: Springer.
- Missouri Botanical Garden n.d. Plant finder: Ampelopsis aconitifolia var. glabra. http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=x330#AllImages
- Soejima, A. & J. Wen 2006. Phylogenetic analysis of the grape family (Vitaceae) based on three chloroplast markers. American Journal of Botany 93(2): 278–287.
- Brickell, C. 2011. American Horticultural Society Encyclopedia of Plants and Flowers. USA: Penguin.
- Bettiga, L.J. 2013. Grape Pest Management, Third Edition, Publication 3343. Richmond, CA: UCANR Publications.
- Mahoney, K. 2002-2014. Latdic. http://www.latin-dictionary.net/definition/20852/folium-foli-i
- Herron, P.M., C.T. Martine, A.M. Latimer, & S.A. Leicht-Young 2007. Invasive plants and their ecological strategies: prediction and explanation of woody plant invasion in New England. Diversity and Distributions 13(5): 633-644.
- Latiff, A. 2012. Seed morphology of Parthenocissus Planch. and Ampelopsis Michx. (Vitaceae) and its taxonomic significance. Sains Malaysiana 41(12): 1503-1508.
- Martine, C.T., S. Leicht-Young, P. Herron, & A. Latimer 2008. Fifteen woody species with potential for invasiveness in New England. Rhodora 110(943): 345-353.
Image Credits (all used with permission):
- Image of leaves and tendril courtesy and copyright, Karl Gercens III
- Image of flowers courtesy of Jörn Germer
- Image of plant with fruits courtesy of Glenn Troester, at B & T World Seeds
PRIMARY AUTHOR: Cristine V. Santanna with revisions and editing by Robyn J. Burnham.
© Robyn J. Burnham
For additional information on Michigan Plant Diversity species accounts, please contact Robyn J. Burnham via email: rburnham“at”umich.edu
Smilax rotundifolia
Cuscuta polygonorum
 Download PDF
Download PDF
 Name: Cuscuta polygonorum Engelmann
Name: Cuscuta polygonorum Engelmann
Family: Convolvulaceae, the morning glory family
Common Names: Dodder, knotweed dodder, smartweed dodder (2,5).
Etymology: With Arabic origins, Kushkut, means dodder plant or parasitic plant; in New Latin, Cuscuta directly translates as dodder. The species name, polygonorum, refers to the species preference for parasitizing species in the genus Polygonum (3,7,16).
Botanical synonyms: Cuscuta chlorocarpa Engelmann (1).
Quick Notable Features (3,4):
¬ Slender orange stems, parasitic
¬ Compact cymose inflroescences, up to 1cm broad
¬ Nearly sessile 4-merous flowers bearing 2 styles shorter than the ovary
¬ Infrastaminal scales with very few teeth, often only 2
Plant Height: The height of Cuscuta polygonorum depends on the host; H.L. Dean measured the length of a single dodder plant at nearly half a mile (9).
Subspecies/varieties recognized: none found.
Most Likely Confused with: Other species of Cuscuta in Michigan: C. indecora, C. cephalanthi, C. coryli, C. glomerata, C. gronovii, and C. pentagona (2).
 Habitat Preference: The species grows mostly on Polygonum ssp., but it is also known to grow on Cephalanthus, Lycopus, Penthorum, Justicia, Asteraceae family members, and other herbs. C. polygonorum prefers low ground in forest thickets, mesic prairies, and wetlands (2,3,6).
Habitat Preference: The species grows mostly on Polygonum ssp., but it is also known to grow on Cephalanthus, Lycopus, Penthorum, Justicia, Asteraceae family members, and other herbs. C. polygonorum prefers low ground in forest thickets, mesic prairies, and wetlands (2,3,6).
Geographic Distribution in Michigan: C. polygonorum is recorded from Kalamazoo, Oakland, and Monroe counties (2).
Known Elevational Distribution: The species was collected at 264m elevation in Holt, MO (1).
Complete Geographic Distribution: Native to North America, the species is found in central and eastern United States: AR, CT, DC, DE, IA, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MO, ND, NE, NJ, NY, OH, OK, PA, RI, TN, TX, VA, and WI; and Canada: ON and QC (1,5).
Parasitism: Plant parasitism is a type of symbiotic relationship in which one plant obtains nutrients directly from a host plant. This has a detrimental effect on the host, but benefits the parasite. Although parasitic plants are commonly known to lack chlorophyll, some species have green tissue, making them partially photoautotrophic. The physical link between the parasite and the host is called a “haustorium,” and often occurs through xylem-to-xylem attachment. The host can vary, ranging from the mycorrihizae of trees, to grasses and hardwood trees. The parasite often maintains open or partially open stomata, allowing transpiration to aid in extracting nutrients from the host (14).
Vegetative Plant Description: As Cuscuta species germinate, they develop a short anchorage root, while a stem forms and nutates (rotates) in search of a host. When an attachment with a host has been created, the anchorage root dies (15). Additional means of finding a host have been suggested in literature, such as positive photoautotrophy or growth toward a source of moisture or specific chemicals (10). The stems of C. polygonorum are slender, filiform, and yellow-orange; they coil around the host plant in a dextral orientation. Leaves are absent, instead there are very small, alternate scales (3,4,8).
Flower Description: The inflorescences are compact cyme heads (glomerules) 0.5-1cm broad bearing nearly sessile, white flowers (2-2.5mm long). The fused calyx is 4-parted (rarely 5) oblong to ovate, and apically acute; both calyx and corolla lobes are at least as long as the corolla tube. The corolla is 4-lobed (rarely 5); the erect lobes are narrower than the sepals, apically acute, and persistent at the base of the fruit. The infrastaminal scales are oblong, with very few teeth, often only two. Four stamens are subtended by the scales and included, adnate to the sinuses of the corolla lobes. The superior ovary is depressed-globose and 2-locular, the two distinct styles (<1mm long) are shorter than the ovary, and the stigmas are capitate (2,3,4,6).
 Flowering Time: July-September (3,4).
Flowering Time: July-September (3,4).
Pollinator: Yuncker observed visits by wasps and other species of the order Hymenoptera to members of the genus Cuscuta (11).
Fruit Type and Description: The fruit is an indehiscent globose capsule, with the remains of the corolla at the base, and the styles at the apex. Each capsule bears up to 4 seeds (3,4).
 Seed Description: In the genus Cuscuta, the defining characteristic of the mature embryo is the absence of cotyledons. This may derive from the fact that the first job of the young stem is to search for a host, not to photosynthesize. Each ovary bears four ovules, but one or more may abort, which causes variation in seed size and shape; a dodder seed may have zero, one, or two flat surfaces. C. polygonorum seeds are about 1.3mm long and yellowish-brown (3,10).
Seed Description: In the genus Cuscuta, the defining characteristic of the mature embryo is the absence of cotyledons. This may derive from the fact that the first job of the young stem is to search for a host, not to photosynthesize. Each ovary bears four ovules, but one or more may abort, which causes variation in seed size and shape; a dodder seed may have zero, one, or two flat surfaces. C. polygonorum seeds are about 1.3mm long and yellowish-brown (3,10).
Dispersal Syndrome: In unspecified members of the genus Cuscuta both germination in the capsule and seeds falling to the ground were observed, leaving water dispersal or other means a possibility for dispersal. Additionally, Cuscuta spp. seeds may be able to pass through the intestinal tract of a sheep intact, remaining viable. Although this method of dispersal is unlikely, it extends the potential dispersal mechanisms to include zoochory (10).
Distinguished by: C. epithymum stems are red, not orange-yellow like in C. polygonorum. It bears 5-merous pink to red flowers with slender stigmas, not white to yellowish with capitate stigmas as in C. polygonorum, and the fruit is circumscissile, while C. polygonorum’s is indehiscent. C. glomerata’s flowers are 5-merous, enclosed by bracts and its sepals are free, while C. polygonorum have bractless flowers with gamosepalous sepals. C. cephalanthi has calyx lobes that are shorter than the corolla tube, the corolla lobes are apically round, and the corolla is persistent at the apex of the capsule. C. polygonorum’s calyx lobes are at least as long as the corolla tube, the corolla lobes are acute, and the corolla is persistent at the base of the capsule. Additionally, the styles in C. cephalanthi are equal or longer than the capsule in length, while in C. polygonorum, they are shorter. C. coryli’s corolla lobes are incurved and papillose, the styles are longer (over 1mm long), and the corolla is persistent at the apex of the capsule. C. polygonorum’s corolla lobes are erect and glabrous, the style shorter than 1mm long. C. gronovii, C. indecora, and C. pentagona are 5-merous. C. gronovii and C. indecora’s ovary and capsule have a thickened stylopodium, not thickened in C. polygonorum. C. gronovii has round corolla lobe apices. C. indecora has papillose corolla lobes, the calyx is shorter than the corolla tube, and the flowers are larger (2.5-4mm long) than the flowers of C. polygonorum. C. pentagona stamens are exserted, while C. polygonorum’s are included (2,3).
Other members of the family in Michigan (number species): Calystegia (5), Convolvulus (1), Cuscuta (8), and Ipomoea (4) (source 2).
Ethnobotanical Uses: The following information is for unspecified members of Cuscuta: “An Indian proverb states that the person finding the root of dodder will have access to all the riches of the earth” (10). This statement pertains to the wide use of Cuscuta spp. for medicinal purposes across Asia, from herbal mixtures to treat ovarian cancer and postmenopausal osteoporosis to antifungal and insecticidal applications (13). From another perspective, “The dodder’s rapid development and its stranglehold on and damage to the host have earned it a place in the superstition of many Western countries. The German “Teufelsxwirn” and Dutch “Duivelsnaaigaren” are vernacular names of this sort,” highlights Cuscuta’s standing as a noxious weed in many places (10).
 Phylogenetic Information: Convolvulaceae joins four other families in the order Solanales (Montiniaceae, Sphenocleaceae, Hydroleaceae, and Solanaceae), which encompasses 165 genera and 4,080 species. The distribution of Convolvulaceae is extensive worldwide, excluding areas of extreme temperatures—the Sahara and Gobi Deserts, and areas of high latitude (Canada, Greenland, Russia, Antarctica, as well as the southern tip of South America). Convolvulaceae has been noted as the only Asterid I family whose seeds exhibit physical dormancy (10). Cuscuta spp., belonging to the subfamily Cuscutoideae, is the only genus within the family that is parasitic. Its placement in Convolvulaceae is openly debated, but is supported by similar flower morphology (10,11,12) as well as the twining habit.
Phylogenetic Information: Convolvulaceae joins four other families in the order Solanales (Montiniaceae, Sphenocleaceae, Hydroleaceae, and Solanaceae), which encompasses 165 genera and 4,080 species. The distribution of Convolvulaceae is extensive worldwide, excluding areas of extreme temperatures—the Sahara and Gobi Deserts, and areas of high latitude (Canada, Greenland, Russia, Antarctica, as well as the southern tip of South America). Convolvulaceae has been noted as the only Asterid I family whose seeds exhibit physical dormancy (10). Cuscuta spp., belonging to the subfamily Cuscutoideae, is the only genus within the family that is parasitic. Its placement in Convolvulaceae is openly debated, but is supported by similar flower morphology (10,11,12) as well as the twining habit.
Interesting Quotation or Other Interesting Factoid not inserted above: Some sources place the genus Cuscuta in its own family, Cuscutaceae (4,5). As other members of the genus Cuscuta, this species is considered a noxious weed in the United States; it is endangered in Maryland and New York (5).
Literature and websites used:
- Tropicos.org. Missouri Botanical Garden. 04 Dec 2012 <http://www.tropicos.org/Name/8500700>
- Michigan Flora Online. A.A. Reznicek, E.G. Voss, & B.S. Walters February 2011. University of Michigan. Web. December 4, 2012. http://michiganflora.net/species.aspx?id=858.
- Fernald, M. L. 1950. Gray’s Manual of Botany, 8th ed. New York: American Book Company.
- Britton, N.L. & H.A. Brown 1970. An Illustrated Flora of the Northern United States and Canada: Volume III. New York, NY: Dover Publications, Inc.
- USDA, NRCS. 2012. The PLANTS Database (http://plants.usda.gov/java/profile?symbol=CUPO, 12/04/12). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- Iverson, L., D. Ketzner, & J. Karnes 2009. Species Information for Cuscuta polygonorum, smartweed dodder. Illinois Plant Information Network. USDA Forest Service. http://nrs.fs.fed.us/data/il/ilpin/spp/?spp=947
- Stearn, W.T. 1972. Stearn’s Dictionary of Plant Names for Gardeners. New York, NY: Sterling Publishing Co. Inc.
- Marquardt, E.S. 2009. Foraging and host use of the parasitic plant Cuscuta indecora.Doctoral Thesis/Dissertation: University of Houston. http://udini.proquest.com/view/foraging-and-host-use-of-the-pqid:1860341511/
- Dean, H.L. 1942. Total length of stem developed from a single seedling of Cuscuta. Proc. Iowa Acad. Sci. 49: 127–128.
- Kuijt, J. 1969. The Biology of Parasitic Flowering Plants. Los Angeles, CA, USA: University of California Press.
- Yuncker, T.G. 1920. Revision of the North American and West Indian Species of Cuscuta. Illinois Botanical Monographs. 6(2&3):1-141.
- Olmstead, R.G. & S. Stefanović 2004. Testing the phylogenetic position of a parasitic plant (Cuscuta, Convolvulaceae, Asteridae): Bayesian inference and the parametric bootstrap on data drawn from three genomes. Systematic Biology 53(3): 384-399.
- Costea, M. & T.J. François 2005. The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. ex Schult., C. umbrosa Beyr. ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Canadian Journal of Plant Science. 298.
- Clark, W.D., R. Moore, & K.R. Stern 1995. Botany. Dubuque, Iowa: Wm. C. Brown Publishers.
- Menninger, E.A. 1970. Flowering Vines of the World. New York, New York: Hearthside Press Incorporated.
- Brown, R.W. 1956. Composition of Scientific Words. Washington, D.C.: Smithsonian Institution Press.
Image Credits (all used with permission):
- Image of plant with inflorescence courtesy of George H. Bruso, Lady Bird Johnson Wildflower Center
- Image of colonization by C. polygonorum courtesy of Diana Lindenmeyer
- Image of immature flowers courtesy of John Hilty from Illinois Wildflowers
- Image of fruiting herbarium specimen courtesy of CONN Herbarium. Copyright © 2012 The George Safford Torrey Herbarium (CONN).
- Species distribution map, derived from the Michigan Flora Online.
PRIMARY AUTHOR:
- Cristine V. Santanna, John Bradtke, and Lauren Sopher, revisions and editing by Robyn J. Burnham.
© Robyn J. Burnham
For additional information on Michigan Plant Diversity species accounts, please contact Robyn J. Burnham via email: rburnham“at”umich.edu
Galium asprellum
 Download PDF
Download PDF
Name: Gallium asprellum Michx.
Family: Rubiaceae, the Madder and Coffee Family
Common Names: Rough bedstraw, kidney-vine, cleavers, clivers (1,7,12).
Etymology: Gala, the Greek word from which Galium is derived, means “milk”. Some species of Galium were used to curdle milk, thus giving the genus this name. The specific epithet, asprellum, means “slightly rough”, referring to the roughness of the leaves and stems (5,9).
Botanical synonyms: None found.
Quick Notable Features (4,6,9):
¬ Herbaceous plant with small hooked prickles that catch on clothing or fur
¬ Most leaves in whorls of 6
¬ Axillary and terminal panicles of small white flowers
¬ Paired, smooth, round capsules
Plant Height: Typically 0.5-2.13 m long (5,9,10).
Subspecies/varieties recognized (7):
G. asprellum var. dahuricum (Turcz. ex Ledeb.) Maxim.
G. asprellum var. fructohispidum Maxim.
G. asprellum var. lasiocarpum Makino
G. asprellum var. tokyoense (Makino) Nakai
G. asprellum var. typicum (Michx.) Maxim.
Most Likely Confused with: Other species of Galium (especially Galium aparine and G. verrucosum), Sherardia arvensis, and Mollugo verticillata.
Habitat Preference: Rough bedstraw prefers wet to moist habitats and it is considered an obligate wetland species. The species is found in swamps, wet thickets, calcareous fens, riparian zones, marshes, wet meadows, bogs, and wet disturbed habitats (1,3,6,10).

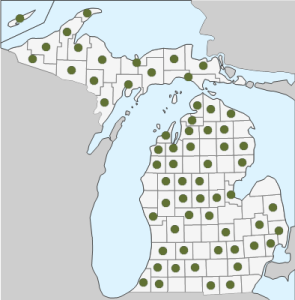
Geographic Distribution in Michigan: G. asprellum is widespread in Michigan, reported in every county in the Upper Peninsula, and most counties in the Lower Peninsula (1).
Known Elevational Distribution: The species grows between 0 and 300 m above sea level. The highest elevation reported was in Alger Co., MI (7).Complete Geographic Distribution: Native to eastern North America. In the United States, G. asprellum is found in every state east of Minnesota and north of North Carolina (except KY), and it was recently reported in Louisiana. In Canada, it is found in NB, NF, NS, ON, PE, and QC (3,13).
 Vegetative Plant Description: G. asprellum is a perennial herbaceous plant growing from creeping rhizomes and producing 4-angled branched stems. The plant stems are erect, prostrate, or leaning on adjacent vegetation, often forming mats. The stems, the margins of leaves, stipules, and the abaxial side of the midribs are covered in retrorse (backward pointing) prickles or bristles. There are 2 opposite leaves and 2-4 leaf-like stipules that form a whorl of 4 to 6 apparent leaves: 6 on the main branches and stem, and sometimes 4 or 5 on branchlets. The leaves and stipules are elliptic to oblanceolate, apically cuspidate, basally attenuate, sessile, entire, 0.5-2.5 cm long, and 0.2-0.5 cm broad (5,6,8,9,17).
Vegetative Plant Description: G. asprellum is a perennial herbaceous plant growing from creeping rhizomes and producing 4-angled branched stems. The plant stems are erect, prostrate, or leaning on adjacent vegetation, often forming mats. The stems, the margins of leaves, stipules, and the abaxial side of the midribs are covered in retrorse (backward pointing) prickles or bristles. There are 2 opposite leaves and 2-4 leaf-like stipules that form a whorl of 4 to 6 apparent leaves: 6 on the main branches and stem, and sometimes 4 or 5 on branchlets. The leaves and stipules are elliptic to oblanceolate, apically cuspidate, basally attenuate, sessile, entire, 0.5-2.5 cm long, and 0.2-0.5 cm broad (5,6,8,9,17).
Climbing Mechanism: G. asprellum climbs by leaning on adjacent vegetation with the aid of its recurved bristles (9,14).
Flower Description: The small perfect flowers of G. asprellum (~ 0.3 cm broad) are borne in axillary and terminal panicles or cymes up to 1.9 cm broad that branch 1-3 times. The inflorescence is longer than the subtending leafy bracts, and the flowers are pedicellate. The calyx is unlobed and fused to corolla and filament bases as a hypanthium. The corolla is 4-parted, white, rotate, each lobe cuspidate and longer than wide. The 4 stamens have short filaments and exserted anthers. The bi-carpellate ovary bears 2 short styles with one capitate stigma each; the carpels bear one ovule each (2,5,6,8,9).
Flowering Time: May-September in northeastern U.S. and adjacent Canada (5,6,9).
 Pollinator: Galium ssp. are pollinated by small bees and flies, attracted by the nectariferous disk on the hypanthium, a shared trait among many members of the Rubiaceae (15,18).
Pollinator: Galium ssp. are pollinated by small bees and flies, attracted by the nectariferous disk on the hypanthium, a shared trait among many members of the Rubiaceae (15,18).
Fruit Type and Description: The fruits of rough bedstraw are paired dry capsules. They are round, indehiscent, smooth (rarely appressed pubescent), black at maturity, and 1-2 mm long and 2-2.5 mm broad. Each capsule bears one seed (1,2,6,8,9).
Seed Description: Galium seeds are convex dorsally, concave proximally and enclose a curved embryo (8).
Dispersal Syndrome: No diaspore dispersal information was found in the literature for the species. Galium mollugo, however, also has smooth fruits, which are commonly dispersed by birds, water, or simply by gravity (16), G. asprellum is likely dispersed in a similar way, due the similarly smooth fruit and moist habitats. On the other hand, vegetative parts of G. asprellum are covered in recurved prickles that may attach to animal fur, which would carry the fruits along.
 Distinguished by: Galium aparine, G. odoratum, G. triflorum, G. circaezans, G. lanceolatum, G. boreale, G. kamtschaticum, and G. pilosum all have bristly ovaries and fruits. The only climbing Galium species that grows in Michigan other than G. asprellum is G. aparine, which can be differentiated by the number of leaves and stipules per whorl (6-8) and their length (up to 8.9 cm long). G. asprellum has smooth ovaries and fruits, and there are usually 4-6 leaves/stipules per whorl, each up to 2.5 cm long. G. verum, G. album, and G. sylvaticum have smooth, mostly erect stems and 6-12 leaves/stipules per whorl while G. asprellum has stems covered in hooked prickles. G. verrucosum has fewer branches than G. asprellum, if branching at all, and its fruits are 3-4 mm long (only 1-2 mm long in G. asprellum). The remaining Galium ssp. in Michigan can be differentiated by the lack of a cuspidate apex on the leaves and stipules. Sherardia arvensis is herbaceous with whorled bristly leaves like Galium, however, its calyx is clearly lobed, and the corolla is blue or pink and funnelform. G. asprellum has an unlobed calyx, and the corolla is white and rotate. Mollugo verticillata is a creeping herb with whorled leaves but differs from G. asprellum by its spatulate basal leaves, flowers with a 5-parted calyx, no corolla, 3 or 5 stamens, and superior 3-carpellate ovary. G. asprellum does not have spatulate basal leaves, the unlobed calyx, 4 stamens, and 2-carpellate ovary are borne on a hypanthium, and the corolla is present and 4-parted (1,8).
Distinguished by: Galium aparine, G. odoratum, G. triflorum, G. circaezans, G. lanceolatum, G. boreale, G. kamtschaticum, and G. pilosum all have bristly ovaries and fruits. The only climbing Galium species that grows in Michigan other than G. asprellum is G. aparine, which can be differentiated by the number of leaves and stipules per whorl (6-8) and their length (up to 8.9 cm long). G. asprellum has smooth ovaries and fruits, and there are usually 4-6 leaves/stipules per whorl, each up to 2.5 cm long. G. verum, G. album, and G. sylvaticum have smooth, mostly erect stems and 6-12 leaves/stipules per whorl while G. asprellum has stems covered in hooked prickles. G. verrucosum has fewer branches than G. asprellum, if branching at all, and its fruits are 3-4 mm long (only 1-2 mm long in G. asprellum). The remaining Galium ssp. in Michigan can be differentiated by the lack of a cuspidate apex on the leaves and stipules. Sherardia arvensis is herbaceous with whorled bristly leaves like Galium, however, its calyx is clearly lobed, and the corolla is blue or pink and funnelform. G. asprellum has an unlobed calyx, and the corolla is white and rotate. Mollugo verticillata is a creeping herb with whorled leaves but differs from G. asprellum by its spatulate basal leaves, flowers with a 5-parted calyx, no corolla, 3 or 5 stamens, and superior 3-carpellate ovary. G. asprellum does not have spatulate basal leaves, the unlobed calyx, 4 stamens, and 2-carpellate ovary are borne on a hypanthium, and the corolla is present and 4-parted (1,8).
Other members of the family in Michigan (number species): Cephalanthus (1), Diodia (1), Galium (19), Houstonia (4), Mitchella (1), Sherardia (1), Stenaria (1) (source 1).
Ethnobotanical Uses: The Choctaw use the whole plant as a diuretic, to treat measles, and to induce perspiration (11). Also known as “kidney-vine”, G. asprellum was used for kidney troubles by rural communities (12). During colonial times, the species was used to curdle milk to make English-style cheeses (15).
 Phylogenetic Information: Galium asprellum is included in the Rubioideae subfamily of the Rubiaceae family, which is in the Gentianales order, a part of the Asterid I clade of Angiosperms. The Gentianales order includes 4 other families: Gentianaceae (closest to Rubiaceae), Loganiaceae, Gelsemiaceae, and Apocynaceae. Members of the Rubiaceae are mostly found in tropical climates, although the family is also represented in other climates and it is spread worldwide except for Polar Regions, and the Sahara and Gobi deserts (4).
Phylogenetic Information: Galium asprellum is included in the Rubioideae subfamily of the Rubiaceae family, which is in the Gentianales order, a part of the Asterid I clade of Angiosperms. The Gentianales order includes 4 other families: Gentianaceae (closest to Rubiaceae), Loganiaceae, Gelsemiaceae, and Apocynaceae. Members of the Rubiaceae are mostly found in tropical climates, although the family is also represented in other climates and it is spread worldwide except for Polar Regions, and the Sahara and Gobi deserts (4).
Interesting Quotation or Other Interesting Factoid not inserted above: G. asprellum is a special concern species in Tennessee (3).
Regarding leaves and stipules of Galium: “There is little doubt that in any whorl the two opposite ‘leaves’, one at any rate of which subtends an axillary shoot, are the true leaves, while the other members at the same node are stipules. Thus, in the case of a six-membered whorl there are two leaves, each of which is provided with two stipules. Where only five or four ‘leaves’ occur in a whorl, it is usually understood that in the first case one, and in the second case both, pairs of stipules have undergone a concrescence [fusion]. If, however, more than six ‘leaves’ are present in a whorl, it is explained that one or more of the original four stipules have undergone chorisis [division during development], resulting in the production of supernumerary members” (17).
Literature and websites used:
- Michigan Flora Online. A.A. Reznicek, E.G. Voss, & B.S. Walters February 2011. University of Michigan. December 28, 2013. http://michiganflora.net/species.aspx?id=2581.
- Chayka, K. 2006-2013. Minnesota Wildflowers: Galium asprellum. http://www.minnesotawildflowers.info/flower/rough-bedstraw
- USDA, NRCS. 2013. The PLANTS Database (http://plants.usda.gov, 12/28/2013). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- Stevens, P.F. Angiosperm Phylogeny Website. Version 13, December 2013 http://www.mobot.org/mobot/research/apweb.
- Robert W. Freckmann Herbarium 2013. Smilax Illinoensis. University of Wisconsin, Stevens Point. http://wisplants.uwsp.edu/scripts/detail.asp?SpCode=GALASP
- Radford, A.E., H.E. Ahles, & C.R. Bell 1968. Manual of the Vascular Flora of the Carolinas. Chapel Hill, North Carolina, USA: The University of North Carolina Press.
- Tropicos.org. Missouri Botanical Garden. 28 Dec 2013 <http://www.tropicos.org/Name/27902719>
- Britton, N.L. & H.A. Brown 1970. An Illustrated Flora of the Northern United States and Canada: Volume I. New York, NY: Dover Publications, Inc.
- Fernald, M.L. 1950. Gray’s Manual of Botany, 8thed. New York: American Book Co.
- Mohlembrock, R.H. 2010. Nelumbonaceae to Vitaceae: Water Lotuses to Grapes. USA: Southern Illinois University Press.
- Campbell, T.N. 1951. Medicinal plants used by Choctaw, Chickasaw, and Creek Indians in the early nineteenth century. Journal of the Washington Academy of Sciences 41(9): 285-290.
- Perkins, A.E. 1929. Colloquial names of Maine plants. Torreya 29(6): 149-151.
- Allen, C.M. 2013. Notes on the identification and distribution of the species of the genus Galium (Rubiaceae) in Louisiana. Journal of the Botanical Research Institute of Texas 7(1): 509-513.
- Dufour, A. 1902. Climbing plants of Ohio. Ohio Journal of Science 2(4): 197-200.
- Lumbard, L.H. 1920. Cleavers. The American Botanist 26(3): 95.
- Mersereau, D. & A. DiTommaso 2002. The biology of Canadian weeds. 121. Galium mollugo L. Can. J. Plant Sci. 83:453–466.
- Takeda, H. 1916. Some points in the morphology of the stipules in the Stellatae, with special reference to Galium. Ann Bot os-30(2): 197-214.
- Zomlefer, W.B. 1994. Guide to Flowering Plant Families. Chapel Hill: The University of North Carolina Press.
Image Credits (all used with permission):
- Image of climbing habit courtesy of Pete Curtis, Minneflora, http://minneflora.com/images/identified/full/rough-bedstraw-galium-asprellum-01.jpg
- Image of the underside of leaves and stem courtesy of Rob Routledge, Sault College, Bugwood.org http://www.forestryimages.org/browse/detail.cfm?imgnum=5475374
- Image of the plant apex with inflorescence courtesy of Russ Schipper http://michiganflora.net/image.aspx?img=13198&id=2581
- Image of flowers close-up courtesy of Rob Routledge, Sault College, Bugwood.org http://www.forestryimages.org/browse/detail.cfm?imgnum=5475372
- Image of fruits courtesy of Dennis Profant, http://fieldbioinohio.blogspot.com
- Species distribution map, derived from the Michigan Flora Online.
PRIMARY AUTHOR: Cristine V. Santanna with revisions and editing by John Bradtke and Robyn J. Burnham.
© Robyn J. Burnham
For additional information on Michigan Plant Diversity species accounts, please contact Robyn J. Burnham via email: rburnham“at”umich.edu