 Download PDF
Download PDF
Name: Galium aparine L.
Family: Rubiaceae, the Coffee and Madder family
Common Names: Catchweed bedstraw, stickywilly, scratchgrass, cleavers, goosegrass, among others (2,14,17).
Etymology: Galium is named after the Greek word for bedstraw, galion. The Greek apairo, which means to “lay hold of” or “seize,” gives the species name aparine, since this species tends to climb and cling to other plants (2).
 Botanical synonyms (13,17):
Botanical synonyms (13,17):
G. agreste var. echinospermum Wallr.
G. austral Reiche
G. charoides Rusby
G. chilense Hook. f.
G. chonosense Clos
G. larecajense Wernham
G. pseudoaparine Griseb.
G. spurium L.
G. spurium var. echinospermum (Wallr.) Hayek
G. spurium var. vaillantii (DC.) Gren. & Godr.
G. spurium var. vaillantii (DC.) G. Beck
G. vaillantii DC.
Quick Notable Features (18):
¬ Square stems
¬ Sessile leaves in whorls of 6-8, hairy adaxially, short spiny hairs along abaxial midrib
¬ Recurved prickles on ridges of stems and leaves
¬ Tiny (~2mm), white, four-parted flowers
Plant Height: Stems to 1.5m long (generally prostrate or scrambling) (1).
Subspecies/varieties recognized (2,17):
G. aparine ssp. spurium (L.) Hartm.
G. aparine var. echinospermum (Wallr.) Farw.
G. aparine var. intermedium (Merr.) Briq.
G. aparine var. microphyllum Clos
G. aparine var. minor Hook.
G. aparine var. pseudoaparine (Griseb.) Speg.
G. aparine var. spurium (L.) W.D.J. Koch
G. aparine var. vaillantii (DC.) Koch
Most Likely Confused with: Other species of Galium, especially G. mollugo and G. triflorum. May also be confused with Mollugo verticillata (18,20).
Habitat Preference: Found in a variety of habitats, but does best in shady, moist, rich organic soil. It is often found in disturbed areas such as fencerows, the edges of woodlands, and high-cut turf, and is a common weed of grains and nursery crops (18).
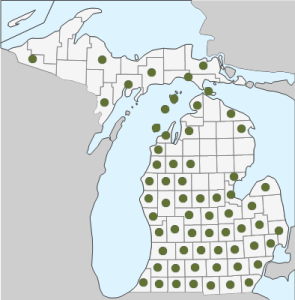
Geographic Distribution in Michigan: Found in 62 of the 83 counties in Michigan—all but Cheboygan, Otsego, Montmorency, Grand Traverse, Kalkaska, Crawford, Oscoda. Alcona, Roscommon, Ogemaw, Iosco, Clare, Gladwin, and Sanilac counties in the Lower Peninsula, and all but Ontonagon, Houghton, Baraga, Iron, Alger, and Luce counties in the Upper Peninsula (17). Although presently found in most counties, G. aparine seems to have been rare in Michigan before 1900, and only grew in the southern Lower Peninsula. However, beginning in the 1940s the plant rapidly spread throughout the state (9).
Known Elevational Distribution: Mostly found at lower elevations in temperate regions such as the UK (15), but in tropical regions it is found at higher elevations (2). The range is quite broad—it is a common understory plant from 1200-1600m on the Inner Mongolian Plateau (6) and important as fodder and in maintaining soils in Himalayan rangelands around 1550m (12).
Complete Geographic Distribution: Although it likely originated in southwest Asia, G aparine is considered native to Europe, Asia, and North America due to its wide prehistoric dispersal. It is now found in all temperate regions, as well as in the warmer polar regions and in high-altitude tropical regions. In the U.S. it is found in every state except Hawaii (2,7,17).
 Vegetative Plant Description: Herbaceous summer or winter annual with a slender taproot and square stems that recline and scramble into “dense, tangled mats that can climb up and over other plants.” The stems have many small, hooked, retrose bristles on each of the four angles, as well as “tufts of hair at the nodes,” and are usually unbranched. Each node has a whorl of apparently 6-8 simple leaves, but in fact the true leaves are accompanied by large stipules (characteristic of Rubiaceae) which are essentially undifferentiable from the leaves. Leaves and stipules are 1-8cm long, 3-10mm wide, and have a papery texture when dried. The leaves have acute bases, are linear to oblanceolate in shape, with acute and shortly mucronate tips. The single prominent midvein comes to a sharp point at the leaf tip, and has many retrose hooked bristles abaxially, as does the margin and upper leaf surface. DeFelice also notes that the species is highly variable genetically. One interesting feature of this plant is that, since only the underside of the leaf has barbs, the undersides of the leaves will catch on fur and clothing, but the upper side will not (pers. obs. RJB) (2,3,8).
Vegetative Plant Description: Herbaceous summer or winter annual with a slender taproot and square stems that recline and scramble into “dense, tangled mats that can climb up and over other plants.” The stems have many small, hooked, retrose bristles on each of the four angles, as well as “tufts of hair at the nodes,” and are usually unbranched. Each node has a whorl of apparently 6-8 simple leaves, but in fact the true leaves are accompanied by large stipules (characteristic of Rubiaceae) which are essentially undifferentiable from the leaves. Leaves and stipules are 1-8cm long, 3-10mm wide, and have a papery texture when dried. The leaves have acute bases, are linear to oblanceolate in shape, with acute and shortly mucronate tips. The single prominent midvein comes to a sharp point at the leaf tip, and has many retrose hooked bristles abaxially, as does the margin and upper leaf surface. DeFelice also notes that the species is highly variable genetically. One interesting feature of this plant is that, since only the underside of the leaf has barbs, the undersides of the leaves will catch on fur and clothing, but the upper side will not (pers. obs. RJB) (2,3,8).
Climbing Mechanism: Recurved prickles on stems and lower leaf surfaces cling to other plants and allow the long stems to scramble and climb (18).
Flower Description: Flowers are tiny: 1.5mm in diameter with 1-30mm pedicels and are borne in groups of 2-5 on axillary cymes, with 4-8 bracts topping the 1-5cm peduncle. The calyx is “a minute annular ridge.” The corolla is radially symmetrical, four-parted, with acute triangular to ovate lobes, and is usually white but can be somewhat yellowish in certain dry habitats. There are four exserted stamens, two short styles, and capitate stigmas. The subglobose 2-carpellate ovary is inferior, 0.3-0.5mm, and bears one ovule per locule; the carpels separate as they mature. The ovary is also covered in small barbs that grow as the fruit develops. The flowers are usually hermaphroditic, but are sometimes andromonoecious (3,4,8,15).
Flowering Time: Generally, late May to late June/mid July, but a few plants flowering as late as September (7,15).
 Pollinator: Although flowers have been observed being visited by a wide range of insects, including Muscid, ichneumon and other flies, small wasps, Lepidoptera, ants, bees (both short- and long-tongued) and beetles, Taylor notes that insects visit flowers only “sparingly.” Additionally, self-pollination is common due to the minute structure of the flower—“when the stigmas mature… they always touch the anthers.” However, the flowers are protandrous, so self-fertilization only occurs if the flower has not already been fertilized by pollen deposited by an insect pollinator (7,15).
Pollinator: Although flowers have been observed being visited by a wide range of insects, including Muscid, ichneumon and other flies, small wasps, Lepidoptera, ants, bees (both short- and long-tongued) and beetles, Taylor notes that insects visit flowers only “sparingly.” Additionally, self-pollination is common due to the minute structure of the flower—“when the stigmas mature… they always touch the anthers.” However, the flowers are protandrous, so self-fertilization only occurs if the flower has not already been fertilized by pollen deposited by an insect pollinator (7,15).
Fruit Type and Description: A 1.4-5mm spherical nutlet ranging in color from greenish or grayish to dark brown, “with a single deep scar on the surface.” The bi-carpellate fruits are covered in hooked bristles; DeFelice also notes that there is a wide range of variation in size and density of bristles (2).
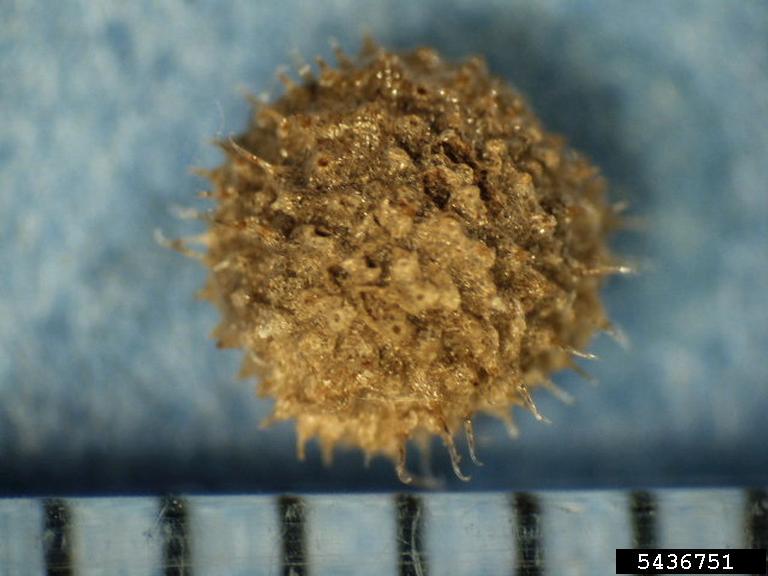 Seed Description: The seeds are kidney-shaped to circular, and have a dense covering of “hooked bristles and tubercles.” They also tend to remain attached to the fruits, and are generally dispersed with the fruit, which does not seem to be dehiscent (1).
Seed Description: The seeds are kidney-shaped to circular, and have a dense covering of “hooked bristles and tubercles.” They also tend to remain attached to the fruits, and are generally dispersed with the fruit, which does not seem to be dehiscent (1).
Dispersal Syndrome: The bristle-covered mericarp of the fruit easily attaches to animals and human clothing, and this kind of dispersal is very effective. The fruits are also buoyant, so water is another dispersal mechanism, as is ingestion by animals, since seeds remain viable after excretion by animals. Additionally, G. aparine seed is a common contaminant of crop seed such as rapeseed, and is also commonly spread this way in agricultural fields (7,15).
Distinguished by: All other Galium species found in Michigan have stems that are glabrous to lightly pubescent, while only G. aparine bears retrose barbs. Additionally, G. aparine has white flowers, as opposed to greenish yellow in G. spurium L. (not documented in MI), and greenish-white in G. triflorum; G. aparine also has larger flowers and fruits than G. spurium. G aparine leaves have retrose/spreading barbs on the margins and are distinctly rougher than G. triflorum, which has antrose barbs, minute bristles or ascending cilia on the margins, with some leaves extremely linear, reduced nearly to the midrib. Although both G. aparine and G. triflorum have leaves in whorls of 6-8, G. aparine mostly has 8 while G. triflorum tends to have 6. Mollugo verticillata is easily distinguished from Galium when in flower by its five- rather than 4-parted flowers with 3 rather than 2 carpels, and when sterile by its smooth, round stems that branch frequently, as opposed to the barbed, square, seldom-branching stems of G. aparine. Sweet woodruff, G. odoratum, is a non-climbing species commonly grown in gardens but seldom escapes (though it does occasionally spread, via rhizome growth, into large clones in shady forests and thickets); it has fragrant foliage and is easily distinguished from G. aparine by its smooth stems (only occasionally with a few spreading hairs) and terminal rather than axillary inflorescences (4,9,18,20).
Other members of the family in Michigan (number species): Cephalanthus (1), Diodia (1), Galium (20), Houstonia (4), Mitchella (1), Sherardia (1), Stenaria (1) (source 9).
Ethnobotanical Uses: Called “bedstraw” because various Galium species were traditionally used to stuff mattresses; the small hooks on the stems made them stick together, resulting in a mattress that retained a uniform thickness longer (11). Also used medicinally in a variety of ways: as a laxative by drinking an infusion, to ease skin irritations by rubbing with a cold infusion of the stem, drinking a decoction of the whole plant for kidney trouble, a compound infusion for hemorrhaging or gonorrhea, and as a hair wash to improve hair growth (10). Seeds can also be roasted and used as a non-caffeinated substitute for coffee—The Great Plains Flora Association notes that it has a reputation of being “the best substitute for coffee in North America” (8,15).
 Phylogenetic Information: There are 611 genera in Rubiaceae. Subclades include Rubioideae (Psychotria, Galium, Spermacoce, Palicourea, Oldenlandia, Hedyotis, Lasianthus, Argostema, Morinda, Gaertnera, Schradera, Margaritopsis), Cinchonoideae (Timonius, Guettarda), Ixoroideae (Pavetta, Ixora, Mussaenda, Coffea, Randia, Tricalysia, Gardenia, Bertiera), and an unnamed clade containing Luculia, Acranthera, and Coptasapelta. Rubiaceae, along with Aponcynaceae, Gelsemiaceae, Gentianaceae, Loganiaceae, and Voyria, is a member of the Gentiales. Gentiales united with the Garryales, Lamiales, Solanales, and a few unplaced clades, forms the Asterid I group of the Asterid clade under the Core Eudicots, which also includes the Rosids (14).
Phylogenetic Information: There are 611 genera in Rubiaceae. Subclades include Rubioideae (Psychotria, Galium, Spermacoce, Palicourea, Oldenlandia, Hedyotis, Lasianthus, Argostema, Morinda, Gaertnera, Schradera, Margaritopsis), Cinchonoideae (Timonius, Guettarda), Ixoroideae (Pavetta, Ixora, Mussaenda, Coffea, Randia, Tricalysia, Gardenia, Bertiera), and an unnamed clade containing Luculia, Acranthera, and Coptasapelta. Rubiaceae, along with Aponcynaceae, Gelsemiaceae, Gentianaceae, Loganiaceae, and Voyria, is a member of the Gentiales. Gentiales united with the Garryales, Lamiales, Solanales, and a few unplaced clades, forms the Asterid I group of the Asterid clade under the Core Eudicots, which also includes the Rosids (14).
Interesting Quotation or Other Interesting Factoid not inserted above:
-The species is a significant yield-reducing weed of cereal crops (21).
-Goodman reports that the stems have a high tensile strength, and the basal region is highly extensible (5); DeFelice, however, describes G. aparine stems as “weak” (2).
-“All the herbe, his branches, leaves, and sede, do cleave and sticke fast to every thing that it toucheth: it is so sharp, that being drawen along the tongue, it wil make it to bleede.” A Neiwe Herball, or Historie of Plantes, D. Rembert Dodoens, 1578 (2).
Literature and websites used:
- Bryson, C.T. & M.S. DeFelice 2009. Weeds of the South. Athens, Georgia: University of Georgia Press.
- DeFelice, M.S. 2002. Catchweed bedstraw or cleavers, Galium aparine L.—a very “sticky” subject. Weed Technology 16 (2): 467-472.
- Chen, T. & F. Ehrendorfer Flora of China, Vol. 19: 28. Galium Linnaeus. http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200022084
- Fernald, M. L. 1950. Gray’s Manual of Botany, 8th ed. New York: American Book Company.
- Goodman, A.M. 2004. Mechanical adaptations of cleavers (Galium aparine). Annals of Botany 95(3): 475-480.
- Liu, H., H. Cui, R. Pott, & M. Speier 2000. Vegetation of the woodland-steppe transition at the southeastern edge of the Inner Monogolian Plateau. Journal of Vegetation Science 11: 525-532.
- Malik, N. & W.H. Vanden Born 1988. The biology of Canadian weeds: Galium aparine L. and Galium spurium L. Canadian Journal of Plant Science 68: 481-499.
- McGregor, R.L. 1986. Flora of the Great Plains. Lawrence, KS: The University Press of Kansas.
- Michigan Flora Online, A.A. Reznicek, E.G. Voss, & B.S. Walters February 2011. University of Michigan. Web. Ret. Jan. 2013. http://michiganflora.net/home.aspx.
- Moerman, D. 2006. Native American Ethnobotany. University of Michigan – Dearborn. http://herb.umd.umich.edu/
- Runkel, S.T. & D.M. Roosa 2009. Wildflowers of the Tallgrass Prairie: The Upper Midwest. Iowa City, IA: University of Iowa Press.
- Singh, V., R.D. Gaur, & B. Bohra 2008. A survey of fodder plants in mid-altitude Himalayan rangelands of Uttarakhand, India. Journal of Mountain Science 5: 265-278.
- Tropicos.org. Missouri Botanical Garden. 19 Feb 2013 <http://www.tropicos.org/Name/27900076>
- Stevens, P.F. Angiosperm Phylogeny Website. Version 12 July 2012. http://www.mobot.org/mobot/research/apweb
- Taylor, K. 1999. Galium aparine L. Journal of Ecology 87: 713-730.
- TWC Staff 2008. Native Plant Database. Lady Bird Johnson Wildflower Center. http://www.wildflower.org/
- USDA, NRCS. 2012. The PLANTS Database (http://plants.usda.gov, ret. Jan. 2013). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- Uva, R., J.C. Neal, & J.M. DiTomaso 1997. Weeds of the Northeast. Ithaca, NY: Cornell University Press.
- Voss, E.G. 1985. Michigan Flora Part II: Dicots. Ann Arbor, MI: Cranbrook Institute of Science.
- Voss, E.G. 2004. Michigan Flora Part III: Dicots Concluded. Ann Arbor, MI: Cranbrook Institute of Science.
- Wilson, B.J. & K.J. Wright 1990. Predicting the growth and competitive effects of annual weeds in wheat. Weed Research 30: 201-211.
Image Credits (all used with permission):
1) Image of plant © T. Beth Kinsey of the Southeastern Arizona Wildflowers
2) Image of flowers and green fruits by Fornax [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
3) Image of fruits © Joseph M. DiTomaso of University of California – Davis and Bugwood.org
4) Image of seed © Bruce Ackley of Ohio State University
5) Species distribution map, derived from the Michigan Flora Online.
Primary Authors: Mackenzie Caple, with revisions and editing by Cristine V. Santanna, John Bradtke, and Robyn J. Burnham.
© Robyn J. Burnham, University of Michigan
For additional information on Michigan Plant Diversity species accounts, please contact Robyn J. Burnham via email: rburnham“at”umich.edu